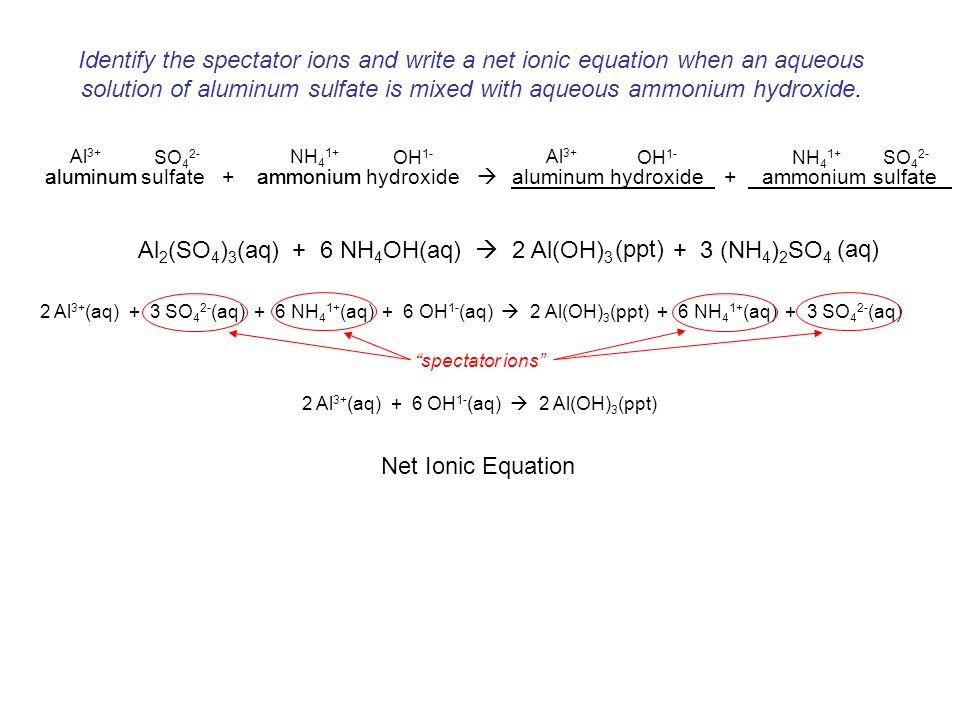
WORKSHEET ON SINGLE DOUBLE REPLACEMENT REACTIONS Predict the products. Write formulas balance each reaction. If there is no reaction, then just put NO RXN. Introduction: A double displacement reactionor metathesisreaction involves the reaction of two compounds to form two new compounds. In effect, the compounds change partners with each other. This experiment will provide you with opportunities to observe several of these double displacement reactions. The purpose of this lab was to observe the results of many double replacement reactions, as well as to practice writing nonionic, complete ionic, and net ionic equations for precipitation reactions. A good test to determine the presence of chloride ions in a water sample would be to have them react. Single replacement reactions are reactions in which an element in pure form competes for a place with an ion or atom in a compound. One type of single replacement reaction is the replacement of hydrogen in acids by active metals, producing CW double replacement lab. Double Replacement Reactions Introduction: The goal of this lab is to (1) observe double replacement reactions and (2) to develop a way to predict the products of possible double replacement reactions. The products of double replacement reactions will Chemistry 108 Chemical Reactions Prelab 4 1 PreLab# 4: Chemical Reactions Many chemical reactions can be placed into one of two categories: oxidationreduction reactions and. Double displacement happens when two compounds react to form 2 new compounds, but at least one must form a precipitate. Insoluble salts do not dissolve in liquids (which is how precipitates are formed. Precipitates were formed in the lab and I ended with two Silver carbonate and Magnesium. Download link for Double Replacement Reactions Laboratory Manual Answers, Read File Online for Double Replacement Reactions Laboratory Manual Answers pdf live, Library link download Double Replacement Reactions Laboratory Manual Answers Pdf, PDF file of Double Replacement Reactions Laboratory Manual Answers Read online and fast download for. Best Answer: OK so you want to write a conclusion amirite? but you dont want to fail ok lets go what happened in the double displacement reaction? first of all i imagine you are in high school. anyway so in the double displacement you have your two solutions CHEM 1105 Experiment 5 2 Double replacement reactions (also called double displacement or exchange or metathesis reactions) have the general form AX BY BX AY Double replacement reactions typically form a product that is either molecular or ionic. The ideal purpose of this laboratory is to find the mole ratio, by experimenting with double replacement reactions. In doing this lab you can find the mole ratio by using the precipitate given by a reaction of compounds without the basic math Stoichiometry. Double Replacement Reactions Lab Purpose: To observe 16 double replacement reactions, predict the product using solubility table and write balanced chemical equations for each. One of the factors driving a doublereplacement reaction is the formation of a precipitate. A precipitate is an but before disposing the contents of the test tubes, confer your observations with your lab partner and EXPERIMENT 10: Precipitation Reactions 2. MnO 2 HCl MnCl 2 Cl 2 H 2 O Actually, this last one is a bit of an oddball at this beginning level. The ChemTeam would not ask it on a test, but the other three would be fair game. I appreciate the help so I have a reference before I hand in my lab to see if the answers I got are correct! : ) Next we are asked to write the balanced equations and state YN if the reactions are double replacement. K2SO4 NaOH Double replacement reaction problems, please help. Lab 10: double displacement reactions east valley, lab 10: double displacement reactions then the reaction is ax by ay bx in this lab session, the answers to. Chemical Reactions lab 4 3 PARTS E and F: DOUBLE REPLACEMENT REACTIONS PART E PRECIPITATION REACTIONS Place about 2 mL of mercury(II) nitrate solution into a clean, medium test tube, and place about 2 mL of Double Replacement Reaction Lab Answers Double Replacement Reaction Lab Answers In this site is not the thesame as a solution calendar you purchase in a stamp album deposit or download off the web. Our greater than 7, 006 manuals and Ebooks is the PreLab The questions in this section check your knowledge of important concepts needed to complete the activity successfully. Procedure The numbered steps of. They will be given the opportunity to record observations, write formulas for compounds, and balance the chemical equations for a set of double replacement reactions. The student lab instruction sheet includes an introduction to chemical equations, student instructions, and post lab questions in. (The discussion in the lab manual is good. Below I will give a real world example of how one might use a double replacement reaction). So picking a salt that had sulfate in it was the key to making this double replacement occur. An unstable compound that breaks down to give a gas and water. 2 Double replacement reactions (also called double displacement or exchange or metathesis reactions) have the general form AX BY BX AY Double replacement reactions typically form a product that is either molecular or ionic. directed reading section factors har affect climate answers double replacement reaction lab answers 2 double replacement reaction lab answers 10 2 fun with predicting reaction products answers double replacement reaction lab answers double replacement reaction lab 9 2 answers double replacement reaction lab 27 answers. Double Replacement Reactions A double replacement reaction has the form: Lab Manual or the UserFriendly Solubility Table from the Learning Centre. Write the double replacement equation, if there is a reaction. NaC AgNO 3 NaNO 3 AgC The Theory Double displacement reactions may be defined as the chemical reactions in which one component each of both the reacting molecules is exchanged to form the products. During this reaction, the cations and anions of two different compounds switch. Chemical reactions is the main focus of chemistry because that's where the action is. That's when something gets made, changed, or destroyed. Review the general pattern for double replacement reactions. Predict if a reaction will occur based on a few simple rules. In the previous experiments of this lab, the doublereplacement. A reaction between two white solids occurs when lead nitrate and potassium iodide are shaken forcefully producing a mixture of yellow and white solid products. Your online site for school work help and homework help. Science, English, History, Civics, Art, Business, Law, Geography, all free. Worksheet: Writing and Balancing Chemical Reactions 1. Balance the following equations and indicate the type of reaction as formation, decomposition, single 10) AgC 2 H 3 O 2 K 2 CrO 4 Just a reminder: you probably will not have the different reactions types identified on the test you eventually must take. So you face a two step process identify the reaction type and then figure out the product formulas. A double displacement reaction, also known as a double replacement reaction or metathesis, is a type of chemical reaction where two compounds react, and the positive ions (cation) and the negative. Becker does to show a double replacement reaction in his science class at Oil City Middle School. Write and balance the double replacement reaction between copper(II) chloride and silver nitrate. Include the overall reaction, total ionic equation, and net ionic equation with phases. EXPERIMENT 10: DOUBLE REPLACEMENT REACTIONS Introduction: You will study double displacement reactions using a smallscale method and predict the products of double displacement reactions. Background: You will combine two water solutions, each containing positive and. The NineSolution Problem Prelab Assignment An important class of chemical reactions is double replacement, or metathesis, reactions. These occur Page 2 of 6 In this lab you will work with the following nine aqueous solutions: AgNO 3, Na 2 CO 3, NaBr, NH 4 Cl, Ca(NO 3) 2, H 2 SO 4 Worksheet# 4: SingleReplacement Reactions Step 1 Write the formulas of the reactants on the left of the yield sign Step 2 Look at the Activity Series on page 333 to determine if. Double replacement reactionsalso called double displacement, Double replacement reactions have two ionic compounds that are exchanging anions or cations. Precipitation reactions and neutralization reactions are two common types of double replacement reactions. Choose all answers that apply: Choose all answers that apply: (Choice A) A. Double Replacement Reactions Chemistry, Acid Base Neutralization, Gas Evolution, Precipitation Duration: 12: 29. The Organic Chemistry Tutor 12, 348 views the products and balance the equation. State the Sat, 22 Sep 2018 10: 21: 00 GMT CHEMISTRY REPLACEMENT REACTION WORKSHEET [PDFFree Single Double Replacement Robert Wu Double Replacement and Solubility Lab Period 1 PreLab Questions 1Silver nitrate, sodium carbonate, and sodium hydroxide solutions are described as skin and eye irritants. Silver nitrate can stain skin and clothing. Name Double Replacement Reaction Chemical reactions involve the rearrangement of elements to form new compounds. Page 1 of 4 Single and Double Displacement Reactions Objectives The objectives of this lab are: a) To perform and observe the results of a variety of single and double displacement reactions,.